

Abstract
The selective reduction of CO2 to liquid fuels by molecular catalysts remains a significant challenge. In this paper, four Ru complexes containing bidentate polypyridyl ligands were synthesized. Each complex also contains a secondary or tertiary amine as part of a benzimidazole moiety. All of the complexes are active in the hydroboration of CO2 to formate, while two of the complexes reduce CO2 to the methoxy oxidation state upon the addition of pinacol borane. 1H and 13C{1H} NMR studies of these chemical reductions suggest the importance of amines in this process, while electrochemical measurements also indicate reactivity with CO2.
Abstract
Photoactivated chemotherapy (PACT) has emerged as a promising strategy to selectively target cancer cells by using light irradiation to generate cytotoxic complexes in situ through a mechanism involving ligand-loss. Due to their rich optical properties and excited state chemistry, Ru polypyridyl complexes have attracted significant attention for PACT. However, studying PACT is complicated by the fact that many of these Ru complexes can also undergo excited-state electron transfer to generate 1O2 species. In order to deconvolute the biological roles of possible photo-decomposition products without the added complication of excited-state electron transfer chemistry, we have developed a methodology to systematically investigate each product individually, and assess the structure-function relationship. Here, we synthesized a series of eight distinct Ru polypyridyl complexes: Ru‑Xa ([Ru(NN)3]2+), Ru-Xb ([Ru(NN)2py2]2+), and Ru-Xc ([Ru(NN)(OH2)2]2+) where NN = 2,2′-bipyridine, 4,4′-dimethyl-2,2′-bipyridine, or dimethyl 2,2′-bipyridine-4,4′-dicarboxylate and py = pyridine. The cytotoxicity of these complexes was investigated in two cell lines amenable to PACT: H23 (breast cancer) and T47D (lung cancer). We confirmed that light irradiation of Ru-Xa and Ru-Xb complexes generate Ru‑Xc complexes through UV-visible spectroscopy, and observed that the Ru-Xc complexes are the most toxic against the cancer cell lines. In addition, we have shown that ligand release and biological activity including bovine serum albumin (BSA) binding, lipophilicity, and DNA interaction are altered when different groups are appended to the bipyridine ligands. We believe that the methodology presented here will enhance the development of more potent and selective PACT agents moving forward.

Abstract
Agrobacterium tumefaciens pathogenesis of plants is initiated with signal reception and culminates with transforming the genomic DNA of its host. The histidine sensor kinase VirA receives and reacts to discrete signaling molecules for the full induction of the genes necessary for this process. Though many of the components of this process have been identified, the precise mechanism of how VirA coordinates the response to host signals, namely phenols and sugars, is unknown. Recent advances of molecular modeling have allowed us to test structure/function predictions and contextualize previous experiments with VirA. In particular, the deep mind software AlphaFold has generated a structural model for the entire protein, allowing us to construct a model that addresses the mechanism of VirA signal reception. Here, we deepen our analysis of the region of VirA that is critical for phenol reception, model and probe potential phenol-binding sites of VirA, and refine its mechanism to strengthen our understanding of A. tumefaciens signal perception.
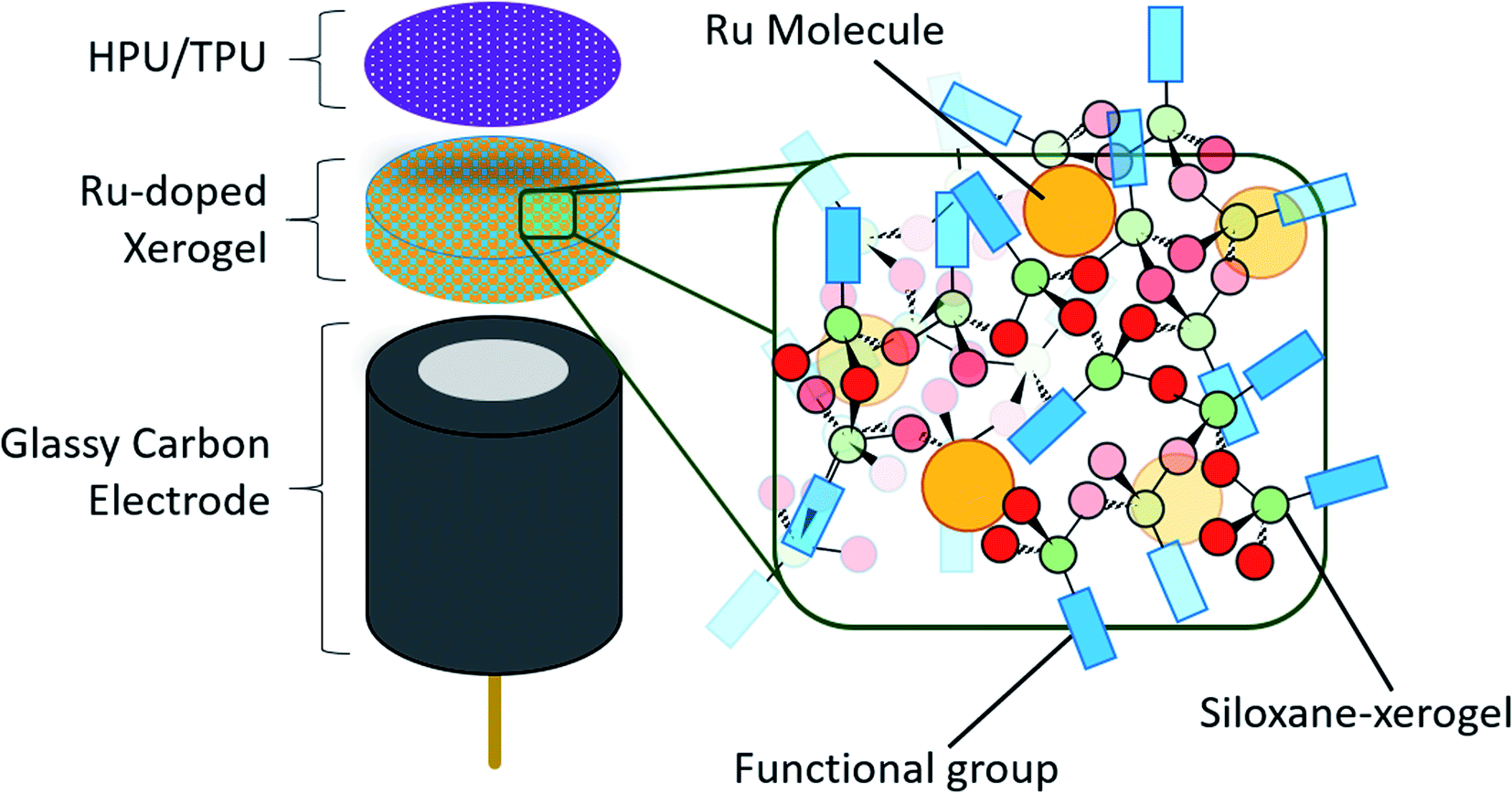
Abstract
The electrochemical and solution stability of discrete molecules trapped in silica-based films on glassy carbon electrodes in aqueous solution was investigated. The films exhibit stability over a wide range of pH values and importantly are created via a simple mixture of commercially available silanes and the electroactive complexes without a need to synthetically modify the target molecules.
-
Brennaman, K. M.; Gish, M. K.; Alibabaei, L.; Norris, M. R.; Binstead, R. A.; Nayak, A.; Lapides, A. M.; Song, W.; Brown, R. J.; Concepcion, J. J.; Templeton, J. L.; Papanikolas, J. M.; Meyer, T. J. "Pathways Following Electron Injection: Medium Effects and Cross-Surface Electron Transfer in a Ruthenium-Based, Chromophore-Catalyst Assembly on TiO2." Journal of Physical Chemistry C. 2018, 122(24), 13017 – 13026.
-
Norris, M. R.; Flowers, S. E.; Mathews, A. M.*; Cossairt, B. M. “H2 Production Mediated by CO2 via Initial Reduction to Formate.” Organometallics. 2016, 35(17), 2778 – 2781.
-
Norris, M. R.; Cossairt, B. M. “CdSe on a Mesoporous Transparent Conducting Oxide Scaffold as a Photocathode.” Journal of Materials Chemistry A (Inside Cover). 2015, 3, 14585 – 14591.
-
Hyde, J.*; Hanson, K.; Vannucci, A.; Lapides, A.; Alibabaei, L.; Norris, M. R.; Meyer, T. J.; Harrison, D. “Electrochemical Instability of Phosphonate-Derivatized, RuIII Polypyridyl Complexes on Metal Oxide Surfaces.” ACS Applied Materials & Interfaces. 2015, 7(18), 9554 – 9562.
-
Brennaman, M.K.; Norris, M. R.; Gish, M. K.; Grumstrub, E. M.; Alibabaei, L.; Ashford, D. L.; Lapides, A. M.; Papanikolas, J. M.; Templeton, J. L; Meyer, T. J. “Ultrafast, Light-Induced Electron Transfer in a Perylene Diimide Chromophore-Donor Assembly on TiO2.” Journal of Physical Chemistry Letters. 2015, 6(23), 4736 – 4742.
-
Alibabaei, L.; Sherman, B. D.; Norris, M. R.; Brennaman, K. M.; Meyer, T. J. “Visible Photoelectrochemical Water Splitting into H2 and O2 in a Dye Sensitized Photoelectrosynthesis Cell.” Proceedings of the National Academy of Sciences. 2015, 112(19), 5893 – 5898.
-
Ashford, D. L.; Glasson, C. R. K.; Norris, M. R.; Concepcion, J. J.; Keinan, S.; Brennaman, M. K.; Templeton, J. L.; Meyer, T. J. “Controlling Ground and Excited State Properties Through Ligand Changes in Ruthenium Polypyridyl Complexes.” Inorganic Chemistry. 2014, 53(11), 5637 – 5646.
-
Alibabaei, L.; Brennaman, K. M.; Norris, M. R.; Kalanyan, B.; Song, W.; Losego, M.; Concepcion, J. J.; Binstead, R. A.; Parsons, G. N.; Meyer, T. J. “Solar Water Splitting in a Molecular Photoelectrochemical Cell.” Proceedings of the National Academy of Sciences. 2013, 110(50), 20008 – 20013.
-
Knauf, R.; Brennaman, K. M.; Alibabaei, L.; Norris, M. R.; Dempsey, J. “Revealing the Relationship Between Semiconductor Electronic Structure and Electron Transfer Dynamics at Metal Oxide-Chromophore Interfaces.” The Journal of Physical Chemistry, C. 2013, 117, 25259 – 25268.
-
Norris, M. R.; Concepcion, J. J.; Fang, Z.; Templeton, J. L.; Meyer, T. J. “Low Overpotential Water Oxidation by a Surface-Bound Ruthenium-Chromophore-Ruthenium-Catalyst Assembly.” Angewadnte Chemie Int. Ed. 2013, 52(51), 13580 – 13583.
-
Norris, M. R.; Concepcion, J. J.; Glasson, C. R. K.; Fang, Z.; Lapides, A. M.; Ashford, D. L.; Templeton, J. L.; Meyer, T. J. “Synthesis of Phosphonic Acid Derivatized Bipyridine Ligands and Their Ruthenium Complexes.” Inorganic Chemistry. 2013, 52(21), 12492 – 12501.
-
Norris, M. R.; Concepcion, J. J.; Harrison, D. P.; Binstead, R. A.; Ashford, D. L.; Fang, Z.; Templeton, J. L.; Meyer, T. J. “Redox Mediator Effect on Water Oxidation in a Ruthenium Based Chromophore-Catalyst Assembly.” Journal of the American Chemical Society. 2013, 135 (6), 2080 – 2083.
-
Giokas, P. G.; Miller, S. A.; Hanson, K.; Norris, M. R.; Glasson, C. R. K.; Concepcion, J. J.; Bettis, S. E.; Meyer, T. J.; Papanikolas, J. M.; Moran, A. M. “Spectroscopy and Dynamics of Phosphonated Ruthenium Complexes on TiO2.” Journal of Physical Chemistry C. 2013, 117 (2), 812 – 824.
-
Wingard, L. A.; Finniss, M. C.; Norris, M. R.; White, P. S.; Brookhart, M.; Templeton, J. L. “Synthesis, Structure, and Reactivity of Iridium(III) Complexes Containing a 4,6-Dimethyl-1,3-benzenediphenylimine Pincer Ligand.” Inorganic Chemistry. 2013, 52 (1), 515 – 526.
-
Hanson, K.; Brennaman, M. K.; Ito, A.; Luo, H.; Song, W.; Parker, K. A.; Ghosh, R.; Norris, M. R.; Glasson, C. R. K.; Concepcion, J. J.; Lopez, R.; Meyer, T. J. “Structure-Property Relationships in Phosphonate-Derivatized, Ru(II) Polypyridyl Dyes on Metal Oxide Surfaces in an Aqueous Environment.” Journal of Physical Chemistry C. 2012, 116(28), 14837 – 14847.
-
Hanson, K.; Torelli, D.*; Vannucci, A.; Brennaman, M. K.; Luo, H.; Alibabaei, L.; Song, W.; Ashford, D. L.; Norris, M. R.; Glasson, C. R. K.; Concepcion, J. J.; Meyer, T. J. “Self-assembled Bilayer Films of Ru(II) Polypyridyl Complexes by Layer-by-Layer Deposition on Nanostructured Metal Oxides.” Angewandte Chemie International Edition. 2012, 51 (51), 12782 – 12785.
-
Ashford, D. L.; Song, W.; Concepcion, J. J.; Glasson, C. R. K.; Brennaman, M. K.; Norris, M. R.; Fang, Z.; Templeton, J. L.; Meyer, T. J. “Photoinduced Electron Transfer in a Chromophore-Catalyst Assembly Anchored to TiO2.” Journal of the American Chemical Society. 2012, 134(46), 19189 – 19198.
-
Song, W.; Chen, Z.; Glasson, C. R. K.; Hanson, K.; Luo, H.; Norris, M. R.; Ashford, D. L.; Concepcion, J. J.; Brennaman, M. K.; Meyer, T. J. “Interfacial Dynamics and Solar Fuel Formation in Dye-Sensitized Photoelectrosynthesis Cells.” ChemPhysChem. 2012, 13(12), 2882 – 2890.
-
Ashford, D. L.; Stewart, D. J.; Glasson, C. R.; Binstead, R. A.; Harrison, D. P.; Norris, M. R.; Concepcion, J. J.; Fang, Z.; Templeton, J. L.; Meyer, T. J. “An Amide-Linked Chromophore-Catalyst Assembly for Water Oxidation.” Inorganic Chemistry. 2012, 51(12), 6428 – 6430.
-
Chen, Z.; Concepcion, J. J.; Brennaman, M. K.; Kang, P.; Norris, M. R.; Hoertz, P. G.; Meyer, T. J. “Splitting CO2 into CO and O2 by a single catalyst.” Proceedings of the National Academy of Sciences of the United States of America, Early Edition. 2012, 1 – 6.
-
Paul, A.; Hull, J. F.; Norris, M. R.; Chen, Z.; Ess, D. H.; Concepcion, J. J.; Meyer, T. J. “Multiple Pathways for Benzyl Alcohol Oxidation by RuV=O3+ and RuIV=O2+.” Inorganic Chemistry. 2011, 50(4), 1167 – 1169.
-
Jurss, J. W.; Concepcion, J. C.; Norris, M. R.; Templeton, J. L.; Meyer, T. J. “Surface Catalysis of Water Oxidation by the Blue Ruthenium Dimer.” Inorganic Chemistry. 2010, 49(9), 3980 – 3982.
-
Concepcion, J. J.; Jurss, J. W.; Norris, M. R.; Chen, Z.; Templeton, J. L.; Meyer, T. J. “Catalytic Water Oxidation by Single-Site Ruthenium Catalysts.” Inorganic Chemistry. 2010, 49(4), 1277 – 1279.
-
Pfennig, B. W.; Norris, M. R.*; Zimmerman, N.*; Gallagher, J. R.*; McCloskey, A. I.* “Synthesis, spectroscopy, electrochemistry, and photochemistry of cyano-bridged mixed-valence coordination compounds containing two different types of intervalent charge-transfer bands.” Inorganica Chimica Acta. 2009, 362(6), 1701 – 1708.
